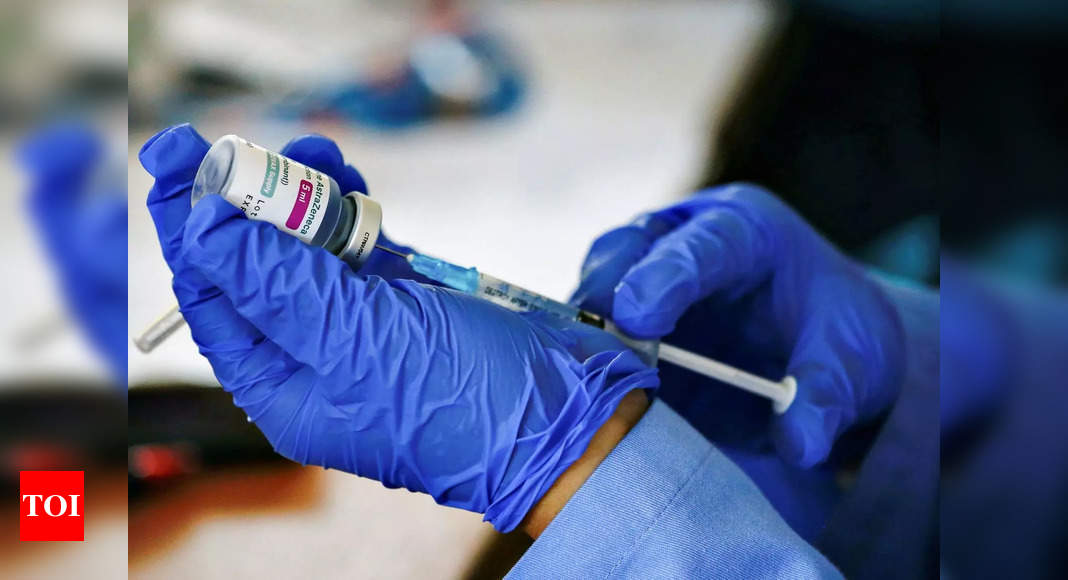
The Covid-19 working group under the government’s vaccine advisory body has recommended use of the Serum Institute of India’s Covovax in the national vaccination programme for those aged 12 years and above, official sources said on Sunday.
The company had requested the ministry to include its vaccine for immunization of adolescents. Based on the working group’s recommendation, the National Technical Advisory Group on Immunisation (NTAGI) may submit its final recommendation to the health ministry soon, further expanding the basket of Covid jabs under the programme.
India’s drug regulator had approved Covovax for restricted use in emergency situations in adults on December 28 and in the 12-17 age group, subject to certain conditions, on March 9.
The working group has now recommended to the standing technical sub-committee of the NTAGI that Covovax be included in the national vaccination programme for those aged 12 years and above, sources said.
India began inoculating children aged 12-14 from March 16. Biological E’s Corbevax is being used to inoculate them, whereas Covaxin is being given to adolescents of 15-17 years since January.
The company had requested the ministry to include its vaccine for immunization of adolescents. Based on the working group’s recommendation, the National Technical Advisory Group on Immunisation (NTAGI) may submit its final recommendation to the health ministry soon, further expanding the basket of Covid jabs under the programme.
India’s drug regulator had approved Covovax for restricted use in emergency situations in adults on December 28 and in the 12-17 age group, subject to certain conditions, on March 9.
The working group has now recommended to the standing technical sub-committee of the NTAGI that Covovax be included in the national vaccination programme for those aged 12 years and above, sources said.
India began inoculating children aged 12-14 from March 16. Biological E’s Corbevax is being used to inoculate them, whereas Covaxin is being given to adolescents of 15-17 years since January.
!function(f,b,e,v,n,t,s) {if(f.fbq)return;n=f.fbq=function(){n.callMethod? n.callMethod.apply(n,arguments):n.queue.push(arguments)}; if(!f._fbq)f._fbq=n;n.push=n;n.loaded=!0;n.version=’2.0′; n.queue=[];t=b.createElement(e);t.async=!0; t.src=v;s=b.getElementsByTagName(e)[0]; s.parentNode.insertBefore(t,s)}(window, document,’script’, ‘https://connect.facebook.net/en_US/fbevents.js’); fbq(‘init’, ‘593671331875494’); fbq(‘track’, ‘PageView’);





More News
Cong to review ‘anti-people laws’, says Ramesh, mum on CAA repeal | India News – Times of India
BJP Scripted Sandeshkhali Conspiracy…: Mamata Banerjees Big Charge After TMC Releases Alleged Sting Video
Ex-Delhi Cong chief Lovely joins BJP for 2nd time, along with 4 others | India News – Times of India